Creating an effective global product launch strategy demands comprehensive and practical knowledge of international and country-specific requirements. Without such insight, the development process becomes vulnerable to oversights and inefficiencies. It is crucial to grasp essential local nuances, including time-to-market considerations, fee structures, local testing and clinical evaluation prerequisites, and market-specific classifications, especially in regions where public information is scarce. Regulatory standards vary across countries and are subject to continuous evolution. Successfully navigating these challenges requires meticulous attention to detail and a proactive approach to comply with global regulatory landscapes.
Fang Consulting’s team offers comprehensive regulatory strategies that outline the registration requirements of the client’s intended global market. We are responsible for monitoring local and country-specific requirements, ensuring that submissions reflect the most current regulations, and providing the client with details for informed decision-making.
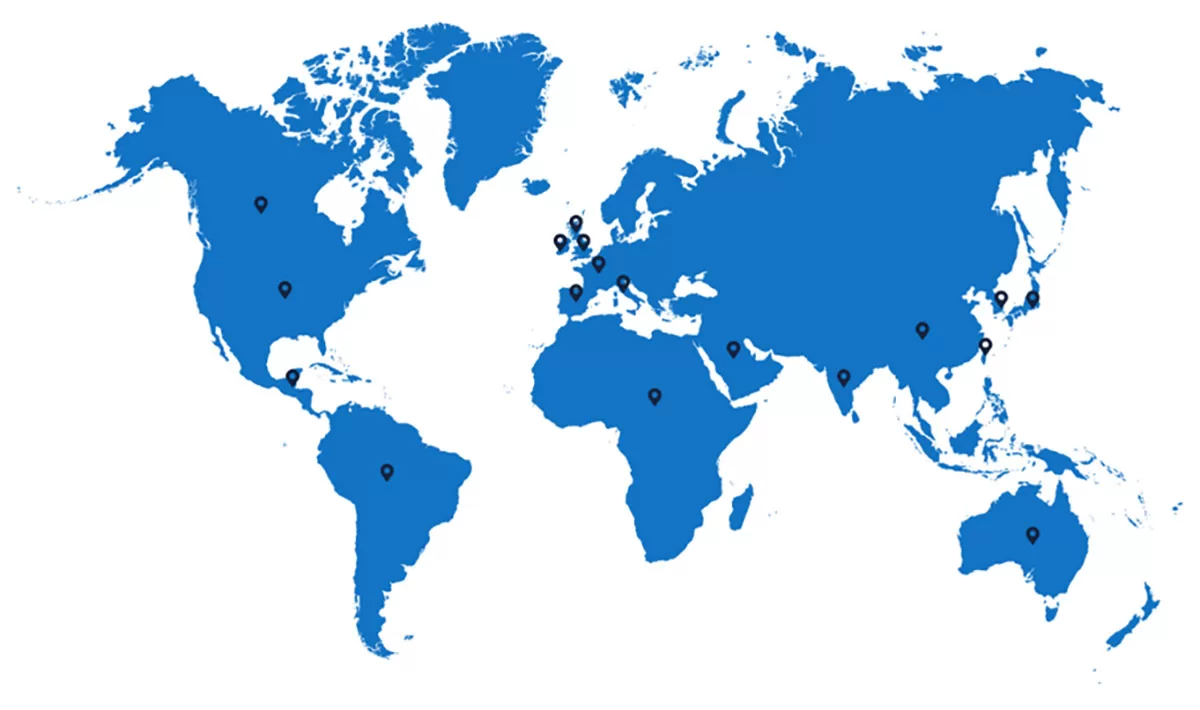
We have successfully helped our clients with hundreds of product registrations in countries such as:
- Canada
- Central & South America
- United Kingdom
- Italy
- Spain
- France
- Ireland
- Germany
- Africa
- Australia
- APAC Countries (China, Japan, South Korea, Taiwan, India)
- And many others
Looking to register your device outside of the United States? Contact us today for a free consultation.